An atomconsists of a positively charged nucleus, surrounded by one or more negatively chargedparticles called electrons. The positive chargesequal the negative charges, so the atom hasno overall charge; it is electrically neutral. The nucleus of an atomcontains protons and neutrons. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. However, the gain or loss of an electron can lead to the formation of an ion, also known as a charged atom. The Charge of Elements.
An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. Most of an atom’s mass is in its nucleus; the mass of an electron is only 1/1836 the mass of the lightest nucleus, that of hydrogen. Although the nucleus is heavy, it is quite small compared with the overall size of an atom.
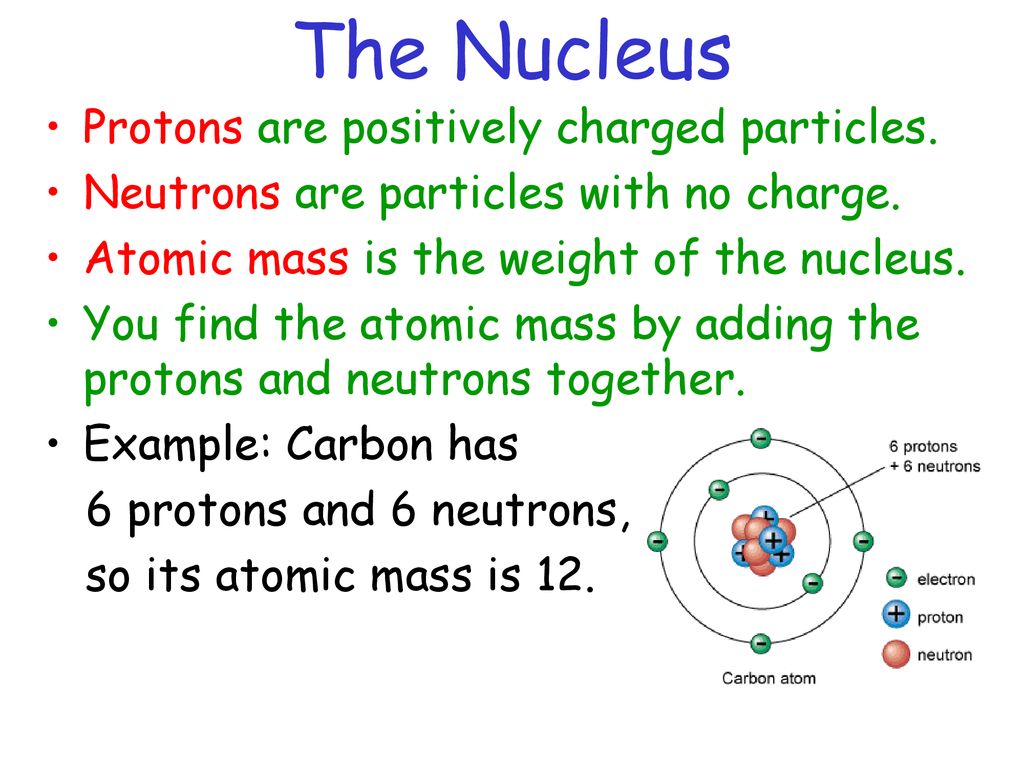
The radius of a typical atom is around 1 to 2.5 angstroms (Å), whereas the radius of a nucleus is about 10-5 Å. If an atom were enlarged to the size of the earth, its nucleus would be only 200 feet in diameter and could easily rest inside a small football stadium. The nucleus of an atom contains protons and neutrons. Protons and neutrons have nearly equal masses, but they differ in charge. A neutron has no charge, whereas a proton has a positive charge that exactly balances the negative charge on an electron. Table (PageIndex{1}) lists the charges of these three fundamental particles, and gives their masses expressed in atomic mass units.
Positively Charged Atoms Are Called
The positively charged part of an atom is the proton, a subatomic particle found in the nucleus. Subsequently, question is, is Atom positive or negative charge? An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. Negatively charged or positively charged atom is generally termed as ANION/CATION. Short Explanation: If an atom loses electrons or gains protons, it will have a net positive charge and is called a Cation. If an atom gains electrons or loses protons, it will have a net negative charge and is called an Anion. A positively charged atom or group of atoms can be called a(n). Anion anode cation cathode.
Positively Charged Atom Or Molecule
Positively Charged Atoms On Periodic Table
| Particle | Charge | Mass (amu) |
|---|---|---|
| Electrons | -1 | 0.000549 |
| Protons | +1 | 1.00782 |
| Neutrons | 0 | 1.00867 |
The atomic mass unit (amu) is defined as exactly one-twelfth the mass of a carbon atom that has six protons and six neutrons in its nucleus. With this scale, protons and neutrons have masses that are close to, but not precisely, 1 u each (there are 6.022 x 1023 u in 1 gram This number is known as Avogadro’s number, N, and one of the ways this number can be calculated is discussed below). The number of protons in the nucleus of an atom is known as the atomic number, Z. It is equal to the number of electrons around the nucleus, because an atom is electrically neutral. The mass number of an atom is equal to the total number of heavy particles: protons and neutrons.
When two atoms are close enough to combine chemically—to form chemical bonds with one another—each atom primarily “sees” the outermost electrons of the other atom. These outer electrons are therefore the most important factors in the chemical behavior of atoms. Neutrons in the nucleus have little effect on chemical behavior, and the protons are significant only because they determine how many electrons surround the nucleus in a neutral atom.
All atoms with the same atomic number behave in much the same way chemically, and are classified as the same chemical element. Each element has its own name and a one- or two-letter symbol (usually derived from the element’s English or Latin name). For example, the symbol for carbon is C, and the symbol for calcium is Ca. The symbol for sodium is Na-the first two letters of its Latin (and German) name, natrium,to distinguish it from nitrogen, N, and sulfur, S.
Example (PageIndex{1}): Bromine
What is the atomic symbol for bromine, and what is its atomic number? Why isn’t the symbol for bromine just the first letter of its name? What other element preempts the symbol B? (Refer to the periodic table)
Solution
Bromine’s atomic number is 35, and its symbol is Br; B is the symbol for boron
Contributors and Attributions
- Dickerson, Richard E. and Gray, Harry B. and Haight, Gilbert P (1979) Chemical principles.
