- Chiral Carbon Center
- Chiral Carbon Practice
- Chiral Carbon Example
- Chiral Carbon Bond
- Chiral Carbons
- Chiral Carbon Atoms Examples
A chiral center is a carbon atom that is chiral at that point. If a molecule has even one chiral center, then it is a chiral molecule. But a molecule can have more than one chiral center, so we. Unless they are placed in a chiral environment. In optical spectroscopy, linearly-polarized light is used to determine the rotation angle in order to determine purity of optically active compounds. Protons attached to chiral centers will give rise to separate signals only if the molecule is placed in a non-chiral environment.
Introduction: Louis Pasteur and the discovery of molecular chirality

In 1848, a 25 year old chemist named Louis Pasteur made a startling – and some thought brash – claim to the scientific community. Pasteur was inexperienced, to say the least: he had only earned his doctorate the previous year, and had just started his first job as an assistant to a professor at the Ecole normale superieure, a university in Paris. Jean-Baptiste Biot, a highly respected physicist who had already made major contributions to scientific fields as diverse as meteorites, magnetism, and optics, was intrigued but unconvinced by Pasteur’s claim. He invited the young man to come to his laboratory and reproduce his experiments.
To Get Full Chapters Visit Our Website: us for any doubts or study-materials at: smarttutorials4you@gmail.comOur aim is to pr. Chiral carbonized polymer dots possesses great advantages in chiral recognition, chiral catalysis, and biology.
(Photo credit: https://www.flickr.com/photos/nate/)
Chiral Carbon Center
Decades earlier, Biot had discovered that aqueous solutions of some biologically-derived substances, such as tartaric acid, quinine, morphine, and various sugars, were optically active: that is, the plane of polarized light would rotate in either a positive (clockwise, or right-handed) or negative (counter-clockwise, or left-handed) direction when passed through the solutions. Nobody understood the source of this optical property. One of the biological substances known to be optically active was a salt of tartaric acid, a compound found in abundance in grapes and a major by-product of the wine-making industry.
The compound was dextrorotatory in solution – in other words, it rotated plane-polarized light in the positive (right-handed, or clockwise) direction. Curiously, though, chemists had also found that another form of processed tartaric acid was optically inactive, despite that fact that it appeared to be identical to the optically active acid in every other respect. The optically inactive compound was called ‘acide racemique‘, from the Latin racemus, meaning ‘bunch of grapes’.
Louis Pasteur’s claims had to do with experiments he said he had done with the ‘racemic’ acid. Jean-Babtise Biot summoned Pasteur to his laboratory, and presented him with a sample of racemic acid which he himself had already confirmed was optically inactive. With Biot watching over his shoulder, and using Biot’s reagents, Pasteur prepared the salt form of the acid, dissolved it in water, and left the aqueous solution in an uncovered flask to allow crystals to slowly form as the water evaporated.
Biot again summoned Pasteur to the lab a few days later when the crystallization was complete. Pasteur placed the crystals under a microscope, and began to painstakingly examine their shape, just as he had done in his original experiments. He had recognized that the crystals, which had a regular shape, were asymmetric: in other words, they could not be superimposed on their mirror image. Scientists referred to asymmetric crystals and other asymmetric objects as being ‘chiral’, from the Greek word for ‘hand’. Your hands are chiral objects, because although your right hand and your left hand are mirror images of one another, they cannot be superimposed. That is why you cannot fit your right hand in a left-handed glove.
More importantly, Pasteur had claimed that the chiral crystals he was seeing under the lens of his microscope were of two different types, and the two types were mirror images of each other: about half were what he termed ‘right handed’ and half were ‘left-handed’. He carefully separated the right and left-handed crystals from each other, and presented the two samples to Biot. The eminent scientist then took what Pasteur told him were the left-handed crystals, dissolved them in water, and put the aqueous solution in a polarimeter, an instrument that measures optical rotation. Biot knew that the processed tartaric acid he had provided Pasteur had been optically inactive. He also knew that unprocessed tartaric acid from grapes had right-handed optical activity, whereas left-handed tartaric acid was unheard of. Before his eyes, however, he now saw that the solution was rotating light to the left. He turned to his young colleague and exclaimed, ” Mon cher enfant, j’ai tant aime ́ les sciences dans ma vie que cela me fait battre le coeur!’ (My dear child, I have loved science so much during my life that this makes my heart pound!)
Biot had good reason to be so profoundly excited. Pasteur had just conclusively demonstrated, for the first time, the concept of molecular chirality: molecules themselves – not just macroscopic objects like crystals – could exhibit chirality, and could be separated into distinct right-handed and left-handed ‘stereoisomers’. Tying together ideas from physics, chemistry, and biology, he had shown that nature could be chiral at the molecular level, and in doing do he had introduced to the world a new subfield which came to be known as ‘stereochemistry’.
About ten years after his demonstration of molecular chirality, Pasteur went on to make another observation with profound implications for biological chemistry. It was already well known that ‘natural’ tartaric acid (the right-handed kind from grapes) could be fermented by bacteria. Pasteur discovered that the bacteria were selective with regard to the chirality of tartaric acid: no fermentation occurred when the bacteria were provided with pure left-handed acid, and when provided with racemic acid they specifically fermented the right-handed component, leaving the left-handed acid behind.
Pasteur was not aware, at the time of the discoveries described here, the details of the structural features of tartaric acid at the molecular level that made the acid chiral, although he made some predictions concerning the bonding patterns of carbon which turned out to be remarkably accurate. In the more than 150 years since Pasteur’s initial tartaric acid work, we have greatly expanded our understanding of molecular chirality. Put simply, stereochemistry is the study of how bonds are oriented in three-dimensional space. It is difficult to overstate the importance of stereochemistry in nature, and in the fields of biology and medicine in particular. As Pasteur so convincingly demonstrated, life itself is chiral: living things recognize different stereoisomers of organic compounds and process them accordingly.

So what, structurally, is a chiral object? The term chiral, from the Greek work for ‘hand’, refers to anything which cannot be superimposed on its own mirror image. Your hands, of course, are chiral – you cannot superimpose your left hand on your right, and you cannot fit your left hand into a right-handed glove (which is also a chiral object). Another way of saying this is that your hands do not have a mirror plane of symmetry: you cannot find any plane which bisects your hand in such a way that one side of the plane is a mirror image of the other side. Chiral objects do not have a plane of symmetry. Your face, on the other hand is achiral – lacking chirality – because, some small deviations notwithstanding, you could superimpose your face onto its mirror image. If someone were to show you a mirror image photograph of your face, you could line the image up, point-for-point, with your actual face. Your face has a plane of symmetry, because the left side is the mirror image of the right side. What Pasteur, Biot, and their contemporaries did not yet fully understand when Pasteur made his discovery of molecular chirality was the source of chirality at the molecular level. It stood to reason that a chiral molecule is one that does not contain a plane of symmetry, and thus cannot be superimposed on its mirror image.
We now know that chiral molecules contain one or more chiral centers, which are almost always tetrahedral carbons with four different substituent groups around them. Consider the cartoon molecule A below: here we have four different substituents denoted by balls of four different colors around a carbon:
The mirror image of A, which we will call B, is drawn on the right side of the figure, and an imaginary mirror is in the middle. Notice that every point on A lines up through the mirror with the same point on B: in other words, if A looked in the mirror, it would see B looking back.
If we flip compound A over and try to superimpose it point for point on compound B, we find that we cannot do it: if we superimpose any two colored balls, then the other two are misaligned.
A is not superimposable on its mirror image (B), thus by definition A is a chiral molecule. It follows that B also is not superimposable on its mirror image (A), and thus it is also a chiral molecule. Neither A nor B has an internal plane of symmetry.
A and B are related to one another as stereoisomers: molecules with the same molecular formula and the same connectivity to other groups, but a different arrangement of atoms in three-dimensional space. There are two types of stereoisomers: enantiomers and diastereomers. Enantiomers are pairs of stereoisomers which are mirror images of each other: thus, A and B are enantiomers. It should be self-evident that a chiral molecule will always have one (and only one) enantiomer: enantiomers come in pairs. Enantiomers have identical physical properties (melting point, boiling point, density, and so on). However, enantiomers do differ in how they interact with polarized light and they may also interact in very different ways with other chiral molecules – proteins, for example. They are different chemical substances, though similar in many ways.
Diastereomers are stereoisomers which are not mirror images of each other. We will come back to diastereomers later.
We defined a chiral center as a tetrahedral carbon with four different substituents. If, instead, a tetrahedral carbon has two identical substituents (two black atoms in the cartoon figure below), then of course it still has a mirror image (everything has a mirror image, unless we are talking about a vampire!) However, it is superimposable on its mirror image, and has a plane of symmetry.
This molecule is achiral. Using the same reasoning, we can see that a carbon with only three groups around it is also not a chiral center.
Notice that structure E can be superimposed on F, its mirror image – all you have to do is pick E up, flip it over, and it is the same as F. This molecule has a plane of symmetry, and is achiral.
Let’s apply our general discussion to real molecules. For now, we will limit our discussion to molecules with a single chiral center. It turns out that tartaric acid, the subject of our chapter introduction, has two chiral centers, so we will come back to it later.
Consider 2-butanol, drawn in two dimensions below.
Carbon #2 is a chiral center: it has four bonds to other atoms and is tetrahedral (even though it is not drawn that way above), and the four things attached to is are different: a hydrogen, a methyl (-CH3) group, an ethyl (-CH2CH3) group, and a hydroxyl (OH) group. Let’s draw the bonding at C2 in three dimensions, and call this structure A. We will also draw the mirror image of A, and call this structure B.
When we try to superimpose A onto B, we find that we cannot do it. A and B are both chiral molecules, and they are enantiomers of each other.
2-propanol, unlike 2-butanol, is not a chiral molecule. Carbon #2 is bonded to two identical substituents (methyl groups), and so it is not a chiral center.
Notice that 2-propanol is superimposable on its own mirror image.
When we look at very simple molecules like 2-butanol, it is not too difficult to draw out the mirror image and recognize that it is not superimposable. However, with larger, more complex molecules, this can be a daunting challenge in terms of drawing and three-dimensional visualization. The easy way to determine if a molecule is chiral is simply to look for the presence of one or more chiral centers: molecules with chiral centers will (almost always) be chiral. We insert the ‘almost always’ caveat here because it is possible to come up with the exception to this rule.
Here’s another trick to make your stereochemical life easier: if you want to draw the enantiomer of a chiral molecule, it is not necessary to go to the trouble of drawing the point-for-point mirror image, as we have done up to now for purposes of illustration. Instead, keep the carbon skeleton the same, and simply reverse the solid and dashed wedge bonds on the chiral carbon: that accomplishes the same thing. You should use models to convince yourself that this is true, and also to convince yourself that swapping any two substituents about the chiral carbon will result in the formation of the enantiomer.
Here are four more examples of chiral biomolecules, each one shown as a pair of enantiomers, with chiral centers marked by red dots.
Here are some examples of achiral biomolecules – convince yourself that none of them contain a chiral center:
Can a chiral center be something other than a tetrahedral carbon with four different substituents? The answer to this question is ‘yes’ – however, these alternative chiral centers are very rare in the context of biological organic chemistry, and outside the scope of our discussion here.
Exercise
Locate all of the chiral centers (there may be more than one in a molecule). Remember, hydrogen atoms bonded to carbon usually are not drawn in the line structure convention – but they are still there!
Exercise
a) Select one of the named substances above that has at least one chiral center. Draw it as a specific stereoisomer. Then draw its enantiomer.
b) Are the two 2-butanol structures below enantiomers?
Exercise
Label the molecules below as chiral or achiral, and locate all chiral centers.
Organic chemistry is one such course that is interesting yet a bit tricky to understand. However, if you are well-versed with all the basic concepts of organic chemistry and familiar with the terms, it becomes a little easy. And so to help you out with it, let us start with the most frequently asked questions regarding chiral carbons and how to find one.
What is chiral carbon?
A carbon atom has the four valence electrons and so it can attach to four atoms at a given time. When such a carbon atom is attached to four different groups at the same time, this carbon atom is known as a chiral carbon or asymmetric carbon. This atom has a center through which it is attached to the four different groups that are referred to as chiral centers. Generally, when the carbon atom bonds to four groups at a given time, these groups are placed in the four corners of the tetrahedron pattern.
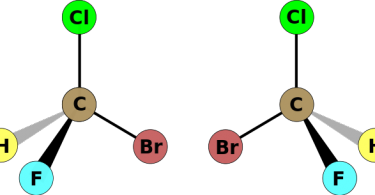
Chiral carbon atoms are also called stereogenic carbons. However, it should be noted that all the functional groups attached to the carbon atom are different from one another. The term chirality means it can’t be a molecule having such property cannot be superimposed.
Chiral Carbon Practice
How to find chiral centers?
As mentioned above, any molecule that has chiral centers when mirrored will not be superimposable. Using this same concept, one can find the chiral centers in the given compound. Chiral atoms, which are also known as asymmetric atoms, have no plane of symmetry. E.g., your left and right hand are mirror images of each other but cannot be superimposed.
Here superimposable refers to when the mirror images, when kept on one another, seem the same. But when any given atom has chiral centers, it will not have superimposable mirror images. Similarly, achiral centers will have superimposed mirror images. And to make it easier for you just look at the four substituents of the carbon atom. If all the four functional groups differ from one another, then it is a chiral atom and that particular carbon atom is the chiral center of the molecule.
For example, CH4 is not a chiral compound because it has the same substituents in all its four positions. Similarly, amino acids, DNA, RNA have chiral centers because they have different functional groups.
Another property that can aid you in finding the chiral centers is their optical activity. The compounds that have chiral carbons are optically active, which means they can bend the plane of polarized light. And based on the direction of rotation, the compounds are either laevorotatory or dextrorotatory, L and D.
Chiral Carbon Example
When the compounds bend the plane of polarization in the right direction when the light is passed through, then it called dextrorotatory. While when it bends the light towards the left direction, it is called laevorotatory.
What are enantiomers?
Chiral Carbon Bond
So by now, you might be knowing that isomerism or isomers mean the atoms with the same molecular formula but with the different spatial arrangement of atoms. These isomers are further classified as:
- Structural isomerism: isomers with same molecular formula but differ in bonded atoms
- Stereoisomerism: isomers with same molecular formula but differ in the orientation of atoms
The compounds that have chiral carbons tend to bend the light in one of the two directions. So a given compound lets say lactic acid can have two isomers L-Lactic acid and D-Lactic acid due to the difference in their orientation. Such stereoisomers that are mirror images of one other but non- superimposable are called enantiomers.
Chiral Carbons
In a nutshell, stereoisomers that are related to each other by reflection are called enantiomers. These isomers will have the same physical property and the chemical formula but only differ in their orientation. One such example of enantiomers is our pair of hands.
Concluding Remarks
Chiral Carbon Atoms Examples
To summarize, chirality means a compound that has mirror images that are not superimposable. The compounds having chiral centers are optically active and bend the light that causes the rotation in the plane of polarization. This rotation decides the orientation of the given compound. And a molecule that has the same chemical formula but different orientation are called enantiomersof each other.
